C4H -- linear butadiynyl radical
|
C4H is a heavy radical molecule with no orbital momentum (say, L = 0) and thus follows momentum coupling scheme of Hund's case b: N + S = J, J + I = F. Here, both electronic spin S = 1/2 and nuclear spin I = 1/2 come from the H atom. As an example, the rotational levels with N = 1 and 2 are shown in Fig. 1. The four main lines (thicker arrows) concentrate about 90% of total transition intensities. (Guelin et al., 1982A&A...109...23G) A list of fine structure transitions were given by Gottlieb et al. (1983ApJ...275..916G). They derived the frequencies by least square fitting to laboratory measurements. |
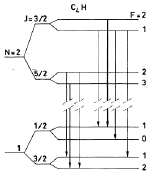
Fig.1 Energy level diagram of N=1 and N=2 rotational levels of C4H showing the seven hyperfine transitions observed in TMC1. The four strongest transitions (main lines) are denoted by thicker lines. (From Guelin et al., 1982A&A...109...23G) |
C4H
Guelin et al., 1986 A&A 157, L17
Cernicharo et al., 1986 A&A 164, L1
Cernicharo et al., 1986 A&A 167, L5
Cernicharo et al., 1987 A&A 172, L5
Cernicharo et al., 1987 A&A 181, L1
Guelin et al., 1987, A&A 175, L5