SiC - Carbon monoxide
| SiC is a open shell molecule. The unpaired electrons make non-zero orbital and spin angular momenta. In electronic ground state, it has a total electronic orbital momentum of L = 1, eletron spin S = 1. The angular momentum coupling follows the Hund's case A: projection of L along the molecular axis is Lambda, Lambda couple with projection of S along the molecular axis to form Omega, then Omega couples with rotational angular momentum to form J. So the quantum number description of levels is (J, Omega, Lambda). There exist three ladders of energy levels 3PI2, 3PI1, and 3PI0 corresponding to three possible Omega values. 16 lines of SiC between 157-276 GHz were observed in laboratory by Cernicharo et al., 1989ApJ...341L..25C. They also detected two transition towards the carbon star IRC +10216. Rotation constants such as A, B, D, gamma, lambda, ..., were determined. | 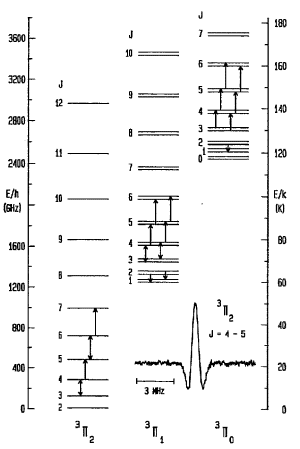 |
| Fig. 1 Energy level diagram in the electronic and vibrational ground state of SiC shown for the three Omega ladders separately. Up arrows are transitions detected in laboratory while down arrows are that detected in space. Shown spectral is a typical profile measured in laboratory. (from Cernicharo et al., 1989ApJ...341L..25C) |